Abstract
Introduction: Venetoclax (Ven) in combination with hypomethylating agent (HMA) is approved for the treatment of newly diagnosed acute myeloid leukemia (AML) in patients 75 years or older or who are not candidates for standard intensive induction chemotherapy (IC) due to comorbidities. Due to the high efficacy of HMA/Ven, an increasing number of newly diagnosed AML patients who may have historically been considered to be candidates for IC are instead receiving HMA/Ven due to patient and/or provider preference, especially patients who are >= 60 years or those with adverse risk disease biology. Several ongoing studies are also evaluating the efficacy of Ven/HMA in newly diagnosed younger AML patients (NCT03573024, NCT04801797, NCT04752527). Although response rates with HMA/Ven are high, approximately 1/3 of patients are refractory and many patients relapse. It is unknown whether salvage IC after Ven/HMA failure is feasible or whether initial therapy with HMA/Ven may impact response to subsequent IC.
Methods: To investigate outcomes of patients treated with IC after failure of frontline HMA/Ven, charts of patients with newly diagnosed AML treated with azacitidine (Aza) + Ven (Aza/Ven) between January 2015 and December 2021 at a single institution were reviewed. Patients that were either refractory to or relapsed on Aza/Ven and received IC (7+3 or other cytarabine-based regimen) as immediate next-line therapy were identified. Baseline patient demographic and clinical information was recorded. The primary endpoint was overall response rate (ORR) with IC after Aza/Ven failure. Secondary endpoints included rate of CR/CRi, the rate of bridging to allogeneic transplant, and overall survival (OS). Responses were assessed according to ELN criteria. An exemption for this retrospective study was obtained from the Institutional Review Board.
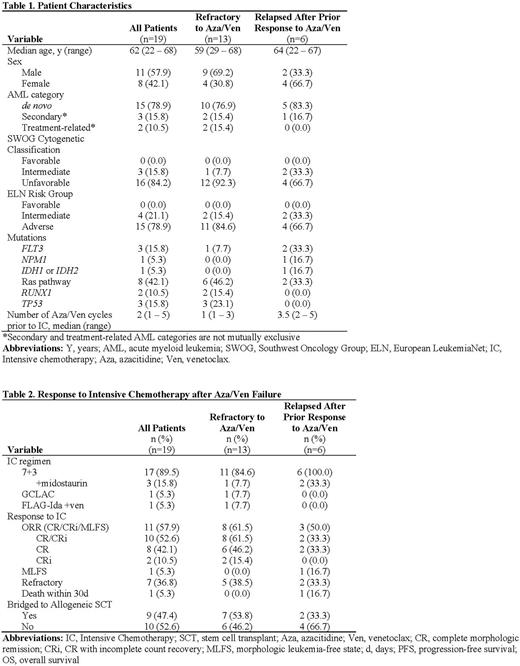
Results: Of 208 patients treated with frontline Aza/Ven, 19 received IC as immediate next-line therapy after Aza/Ven failure, including 13 patients who were treated with IC after their AML was refractory to Aza/Ven up front and 6 patients who received IC after relapsing following a prior response to first-line Aza/Ven. The median age of this cohort was 62 years (range 22 to 68 years) (Table 1). Most patients had unfavorable cytogenetics (16/19, 84.2%) and ELN adverse risk disease (15/19, 78.9%). The median number of cycles of Aza/Ven received prior to IC was 2 (range 1 to 5 cycles). Regarding IC regimen selected, 17 patients received 7+3, including 3 where midostaurin was added due to the presence of a FLT3 mutation, 1 patient received GCLAC, and 1 received FLAG-Ida+venetoclax. Among all patients, the ORR with IC was 57.9% (11/19) (Table 2). The rate of CR/CRi was 52.6% (10/19). Seven patients (36.8%) had AML that was refractory to IC, and 1 subject died within 30 days of IC. Of the 13 patients who had AML that was refractory to Aza/Ven up front, the CR/CRi rate was 61.5% (8/13), including 6 patients who achieved a CR and 2 who achieved a CRi. Of the 11 patients who responded to IC, 9 (81.8%) proceeded to allogeneic stem cell transplant. One patient declined transplant, and 1 transitioned to hospice due to a progressing fungal infection.
Conclusions: For a select patient population, salvage IC after Aza/Ven failure is feasible. In our cohort of patients with predominantly adverse risk disease biology who received IC after failure of Aza/Ven, the ORR with IC was 57.9%, which compares favorably to response rates observed with frontline IC. Whether Aza/Ven and IC targeted different subclones and represent complementary sequential therapies in this high-risk group of AML patients is an ongoing correlative investigation. Overall, this data suggests that initial therapy with HMA/Ven in older and/or adverse risk AML patients with a plan to proceed with IC as second line therapy in the event of Aza/Ven failure is a reasonable strategy in select patients.
Disclosures
McMahon:Arcellx: Consultancy; Syros: Research Funding. Smith:AML JV: Research Funding; Argenx: Research Funding; Syros: Research Funding; Kura: Research Funding. Pollyea:Bristol-Myers Squibb: Consultancy; Aprea: Consultancy; Astellas: Consultancy; Teva: Research Funding; Celgene: Consultancy; Genentech: Consultancy; Foghorn: Consultancy; Kiadis: Consultancy; Gilead Sciences: Consultancy; Syros: Consultancy; Syndax: Consultancy; Novartis: Consultancy; Takeda Oncology: Consultancy; AbbVie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal